Nickel Dmg Structure
- Nickel Dmg Structure Chart
- Nickel Dmg Structure Definition
- Nickel Dmg Structure Formula
- Nickel Dmg Structure System
- Nickel Dmg Structure Diagram
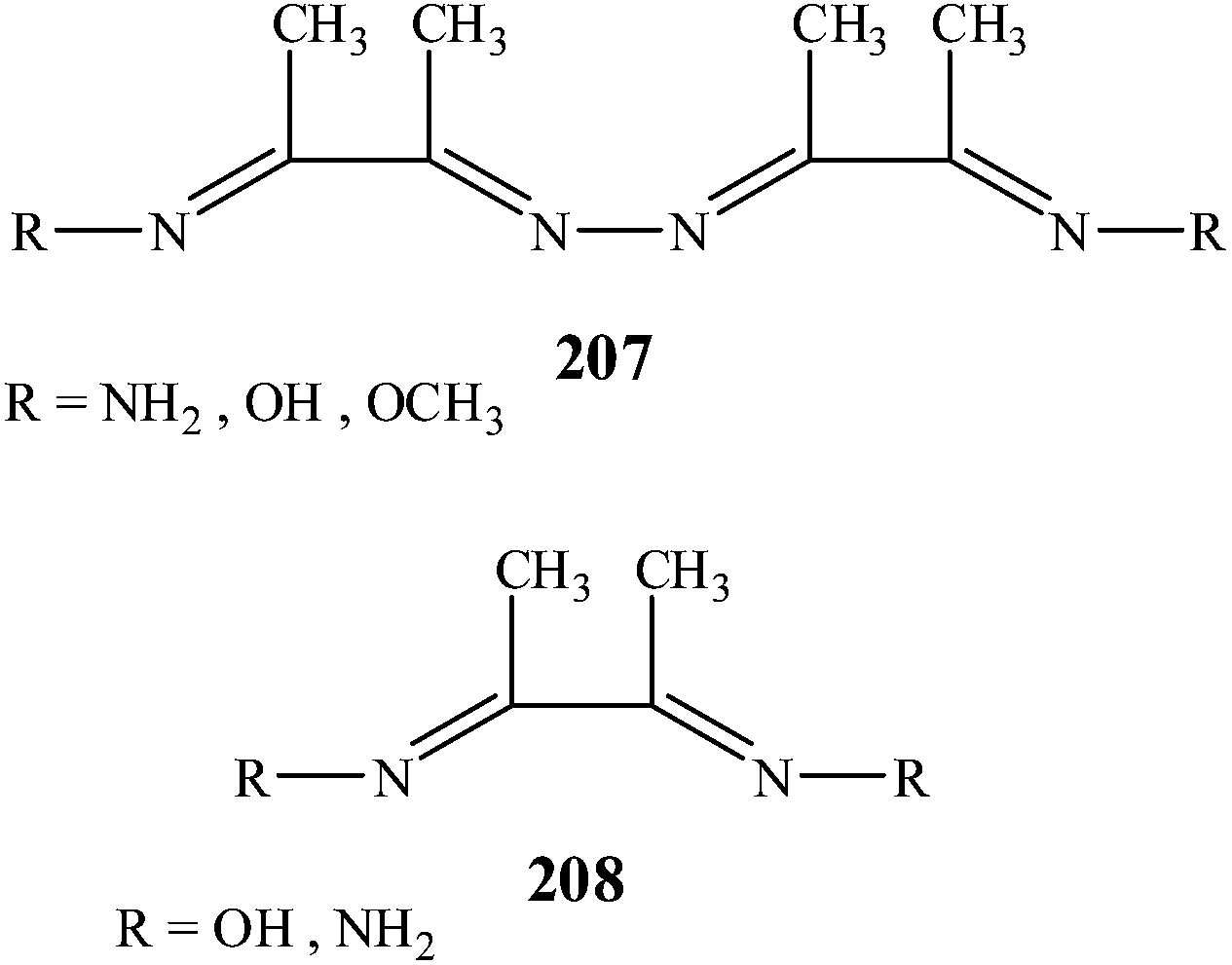
Nickel is precipitated with an organic precipitating agent termed dimethylglyoxime (DMG) as shown below. Organic reagents often react with more than one metal ion, therefore, adequate specification can be achieved with concentration and pH. DMG forms a chelating complex with the. Nickel;N-(Z)-3-nitrosobut-2-en-2-ylhydroxylamine C8H16N4NiO4 CID 5475696 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Nickel-dimethylglyoxime complex (abbreviated as Ni(II)(DMG) 2) modified carbon paste and graphite electrodes were prepared by mixing Ni(II)(DMG) 2 with graphite paste, and coating Ni(II)(DMG) 2 to the graphite surface. It is necessary to cycle the electrode potential to a high value (e.g. 0.8 V versus SCE) for the preparation of the modified electrodes. Its abbreviation is dmgH2 for neutral form, and dmgH for anionic form, where H stands for hydrogen. This colourless solid is the di oxime derivative of the diketone butane-2,3-dione (also known as diacetyl ). DmgH 2 is used in the analysis of palladium or nickel. Its coordination complexes are of theoretical interest as models. SOLUBILITY BEHAVIOR OF SOME COPPER(II)-AND NICKEL(II)-VIC-DIOXIMES by-John Edwin Caton, Jr. A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject; Analytical Chemistry Charge of Major Work Approved: Head of Major Department Iowa State University.
| Names | |
|---|---|
| IUPAC name nickel;N-[(Z)-3-nitrosobut-2-en-2-yl]hydroxylamine | |
| Other names | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| EC Number | |
PubChemCID | |
| |
| |
| Properties | |
| C8H14N4NiO4 | |
| Molar mass | 288.917 g·mol−1 |
| Appearance | red solid |
| Density | 1.698 g/cm3 |
| Hazards | |
| GHS pictograms | |
| GHS Signal word | Warning |
| H315, H317, H319, H335, H351 | |
| P201, P202, P261, P264, P271, P272, P280, P281, P302+352, P304+340, P305+351+338, P308+313, P312, P321, P332+313, P333+313, P337+313, P362, P363, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nickel bis(dimethylglyoximate) is the coordination complex with the formula Ni[ONC(CH3)C(CH3)NOH]2. The compound is a bright red solid. It achieved prominence for its use in the qualitative analysis of nickel.[1]
Structure[edit]
Nickel(II) is square planar.[2] It is surrounded by two equivalents of the conjugate base (dmgH−) of dimethylglyoxime (dmgH2). The pair of organic ligands are joined through hydrogen bonds to give a macrocyclic ligand. The complex is distinctively colored and insoluble leading to its use as a chelating agent in the gravimetric analysis of nickel.
The use of dimethylglyoxime as a reagent to detect nickel was reported by L. A. Chugaev in 1905.[3]
References[edit]
- ^Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN978-0-08-037941-8.
- ^Donald E. Williams, Gabriele Wohlauer, R. E. Rundle (1959). 'Crystal Structures of Nickel and Palladium Dimethylglyoximes'. J. Am. Chem. Soc. 81: 755–756. doi:10.1021/ja01512a066.CS1 maint: uses authors parameter (link)
- ^Lev Tschugaeff (1905). 'Über ein neues, empfindliches Reagens auf Nickel'. Berichte der deutschen chemischen Gesellschaft. 38 (3): 2520–2522. doi:10.1002/cber.19050380317.
| Names | |
|---|---|
| IUPAC name | |
| Other names Nickel hydroxide, Theophrastite | |
| Identifiers | |
| |
| ChemSpider | |
| ECHA InfoCard | 100.031.813 |
| EC Number |
|
| RTECS number | |
CompTox Dashboard(EPA) | |
| |
| |
| Properties | |
| Ni(OH)2 | |
| Molar mass | 92.724 g/mol (anhydrous) 110.72 g/mol (monohydrate) |
| Appearance | green crystals |
| Density | 4.10 g/cm3 |
| Melting point | 230 °C (446 °F; 503 K) (anhydrous, decomposes) |
| 0.13 g/L | |
| +4500.0·10−6 cm3/mol | |
| Structure[1] | |
| hexagonal, hP3 | |
| P3m1, No. 164 | |
α = 90°, β = 90°, γ = 120° | |
| Thermochemistry | |
| 79 J·mol−1·K−1[2] | |
Std enthalpy of formation(ΔfH⦵298) | −538 kJ·mol−1[2] |
| Hazards | |
| Safety data sheet | External SDS |
| GHS pictograms | [3] |
| GHS Signal word | Danger[3] |
| H302, H332, H315, H334, H317, H341, H350, H360, H372[3] | |
| P260, P284, P201, P280, P405, P501[3] | |
| Lethal dose or concentration (LD, LC): | |
| 1515 mg/kg (oral, rat) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| verify (what is ?) | |
| Infobox references | |
Nickel(II) hydroxide is the inorganic compound with the formula Ni(OH)2. It is an apple-green solid that dissolves with decomposition in ammonia and amines and is attacked by acids. It is electroactive, being converted to the Ni(III) oxy-hydroxide, leading to widespread applications in rechargeable batteries.[4]
Properties[edit]
Nickel(II) hydroxide has two well-characterized polymorphs, α and β. The α structure consists of Ni(OH)2 layers with intercalated anions or water.[5][6] The β form adopts a hexagonal close-packed structure of Ni2+ and OH− ions.[5][6] In the presence of water, the α polymorph typically recrystallizes to the β form.[5][7] In addition to the α and β polymorphs, several γ nickel hydroxides have been described, distinguished by crystal structures with much larger inter-sheet distances.[5]
The mineral form of Ni(OH)2, theophrastite, was first identified in the Vermion region of northern Greece, in 1980. It is found naturally as a translucent emerald-green crystal formed in thin sheets near the boundaries of idocrase or chlorite crystals.[8] A nickel-magnesium variant of the mineral, (Ni,Mg)(OH)2 had been previously discovered at Hagdale on the island of Unst in Scotland.[9]
This means that if you don't like the direction you took on a document, or thought a past version was truly what you wanted, you'll now have the ability to pick a better version from the past. Like other new technologies in OS X Lion, versions will only work on core apps like Preview, TextEdit, and the iWork suite initially, but it will be available as an API for third-party developers to add into their own apps, and we suspect most of them will.Along with autosave and versions, you also never have to worry about closing down your Mac in a rush. Autosave and versions is truly a welcome addition to OS X Lion that just about anyone will appreciate. Mac os x lion 10.7 download.
Reactions[edit]
Nickel Dmg Structure Chart
Nickel(II) hydroxide is frequently used in electrical car batteries.[6] Specifically, Ni(OH)2 readily oxidizes to nickel oxyhydroxide, NiOOH, in combination with a reduction reaction, often of a metal hydride (reaction 1 and 2).[10]
Reaction 1 Ni(OH)2 + OH− → NiO(OH) + H2O + e−
Nickel Dmg Structure Definition
Reaction 2 M + H2O + e− → MH + OH−
Nickel Dmg Structure Formula
Net Reaction (in H2O)Ni(OH)2 + M → NiOOH + MH
Of the two polymorphs, α-Ni(OH)2 has a higher theoretical capacity and thus is generally considered to be preferable in electrochemical applications. However, it transforms to β-Ni(OH)2 in alkaline solutions, leading to many investigations into the possibility of stabilized α-Ni(OH)2 electrodes for industrial applications.[7]
Synthesis[edit]
The synthesis entails treating aqueous solutions of nickel(II) salts with potassium hydroxide.[11]
Toxicity[edit]
The Ni2+ ion is a known carcinogen. Toxicity and related safety concerns have driven research into increasing the energy density of Ni(OH)2 electrodes, such as the addition of calcium or cobalt hydroxides.[4]
See also[edit]
References[edit]
- ^Enoki, Toshiaki; Tsujikawa, Ikuji (1975). 'Magnetic Behaviours of a Random Magnet, NipMg(1-p)(OH2)'. Journal of the Physical Society of Japan. 39 (2): 317. doi:10.1143/JPSJ.39.317.
- ^ abZumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN978-0-618-94690-7.
- ^ abcd'Nickel Hydroxide'. American Elements. Retrieved 2018-08-30.
- ^ abChen, J.; Bradhurst, D.H.; Dou, S.X.; Liu, H.K. (1999). 'Nickel Hydroxide as an Active Material for the Positive Electrode in Rechargeable Alkaline Batteries'. J. Electrochem. Soc. 146 (10): 3606–3612. doi:10.1149/1.1392522.
- ^ abcdOliva, P.; Leonardi, J.; Laurent, J.F. (1982). 'Review of the structure and the electrochemistry of nickel hydroxides and oxy-hydroxides'. Journal of Power Sources. 8 (2): 229–255. doi:10.1016/0378-7753(82)80057-8.
- ^ abcJeevanandam, P.; Koltypin, Y.; Gedanken, A. (2001). 'Synthesis of Nanosized α-Nickel Hydroxide by a Sonochemical Method'. Nano Letters. 1 (5): 263–266. doi:10.1021/nl010003p.
- ^ abShukla, A.K.; Kumar, V.G.; Munichandriah, N. (1994). 'Stabilized α-Ni(OH)2 as Electrode Material for Alkaline Secondary Cells'. J. Electrochem. Soc. 141 (11): 2956–2959. doi:10.1149/1.2059264.
- ^Marcopoulos, T.; Economou, M. (1980). 'Theophrastite, Ni(OH)2, a new mineral from northern Greece'(PDF). American Mineralogist. 66: 1020–1021.
- ^Livingston, A.; Bish, D. L. (1982). 'On the new mineral theophrastite, a nickel hydroxide, from Unst, Shetland, Scotland'(PDF). Mineralogical Magazine. 46 (338): 1. doi:10.1180/minmag.1982.046.338.01.
- ^Ovshinsky, S.R.; Fetcenko, M.A.; Ross, J. (1993). 'A nickel metal hydride battery for electric vehicles'. Science. 260 (5105): 176–181. doi:10.1126/science.260.5105.176. PMID17807176.
- ^Glemser, O. (1963) 'Nickel(II) Hydroxide' in 'Handbook of Preparative Inorganic Chemistry, 2nd ed. G. Brauer (ed.), Academic Press, NY. Vol. 1. p. 1549.
External links[edit]
Nickel Dmg Structure System